Plasma is the Fourth State of Matter…
Plasma Classification
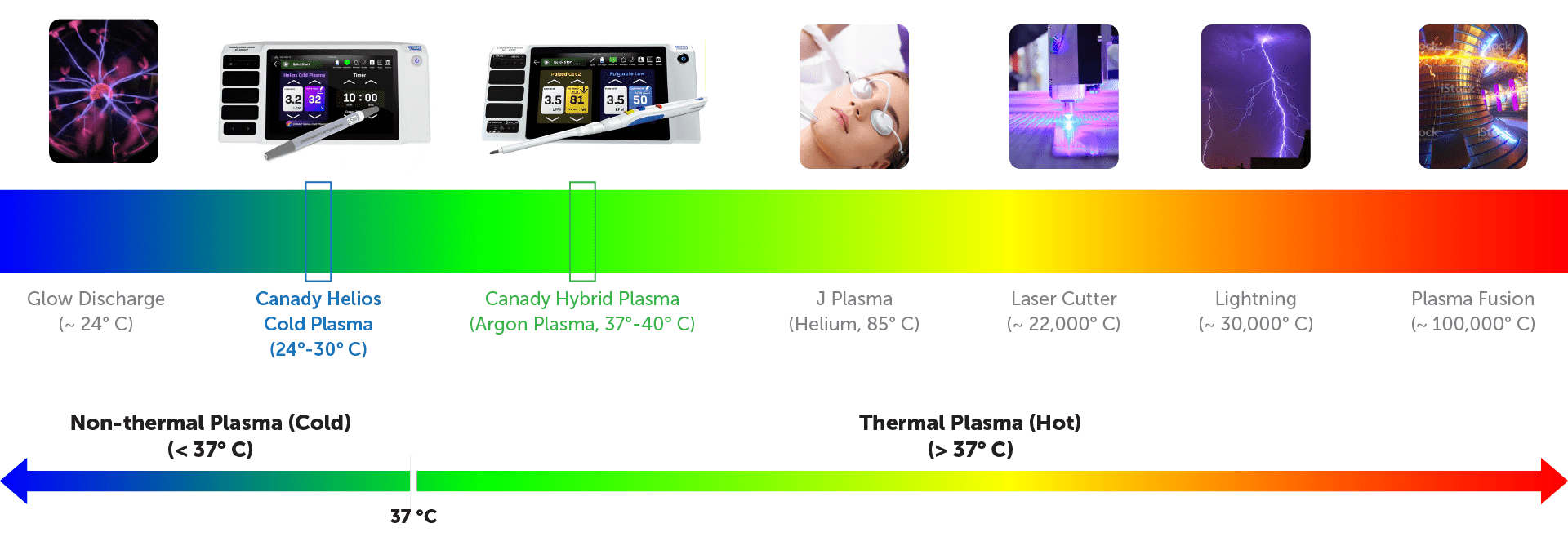
What is Canady Helios™ Cold Plasma (CHCP)?
The Canady Cold Plasma spray is a non-thermal process that triggers chemical-molecular changes in cancer cells, leading to Apoptosis (Cell Death) while healthy human tissue remains unharmed.1

Canady Helios™ Cold Plasma offers Key Benefits to your Patients
- Only One Application Required
- The Plasma Application Occurs During your Surgery and only takes 5-7 minutes
- Safe – No Side Effects

The Application of Canady Helios™ Cold Plasma
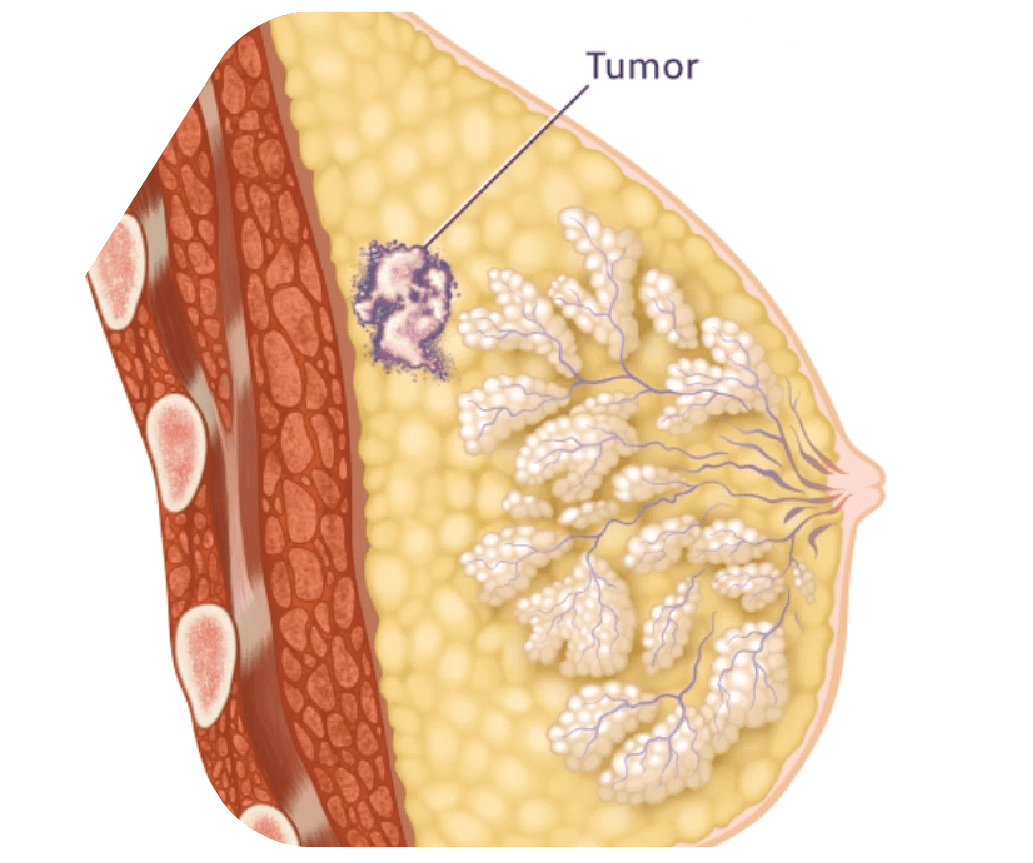
STEP 1
Cancerous Tumor Located in Breast
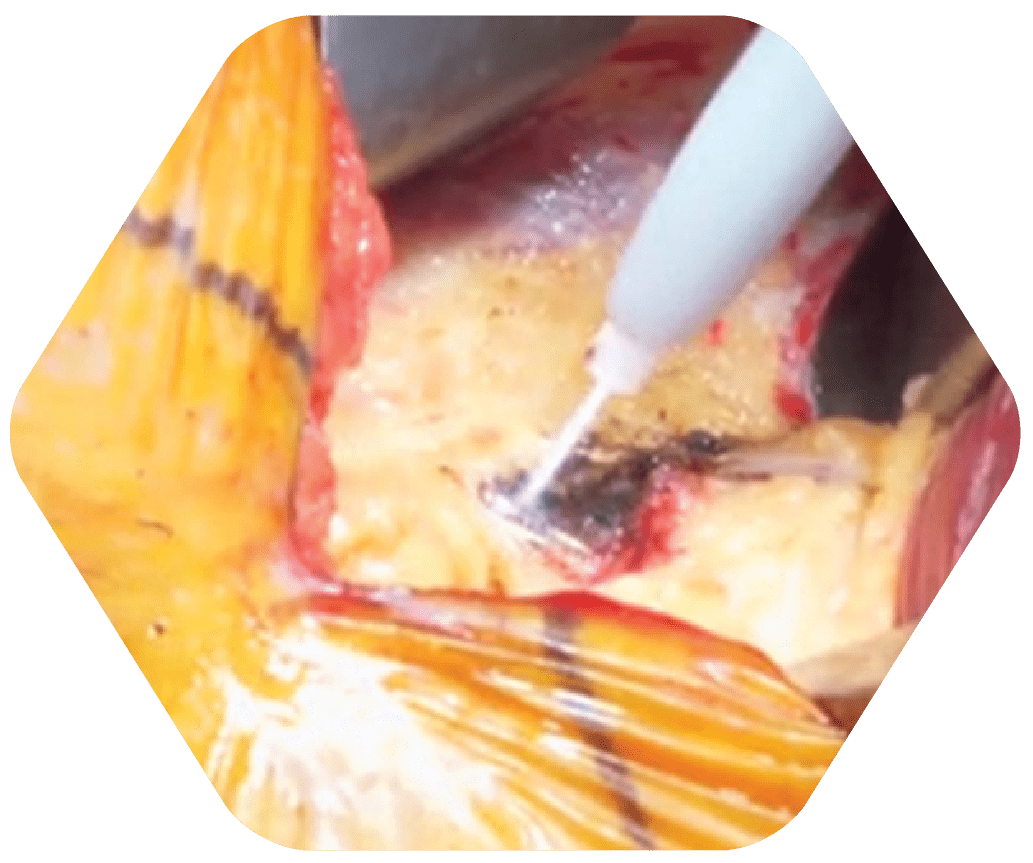
STEP 2
Tumor Removed with Canady Hybrid Plasma Scalpel
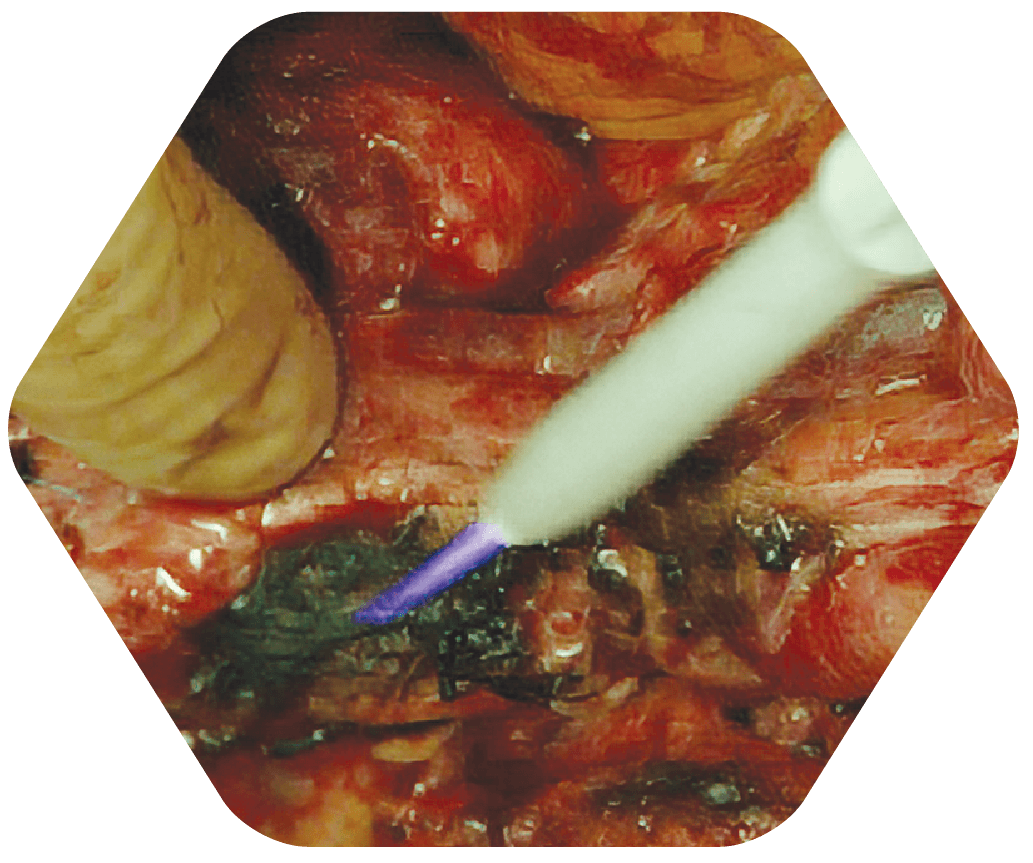
STEP 3
Tumor Margins Sprayed with Canady Helios Cold Plasma (CHCP)
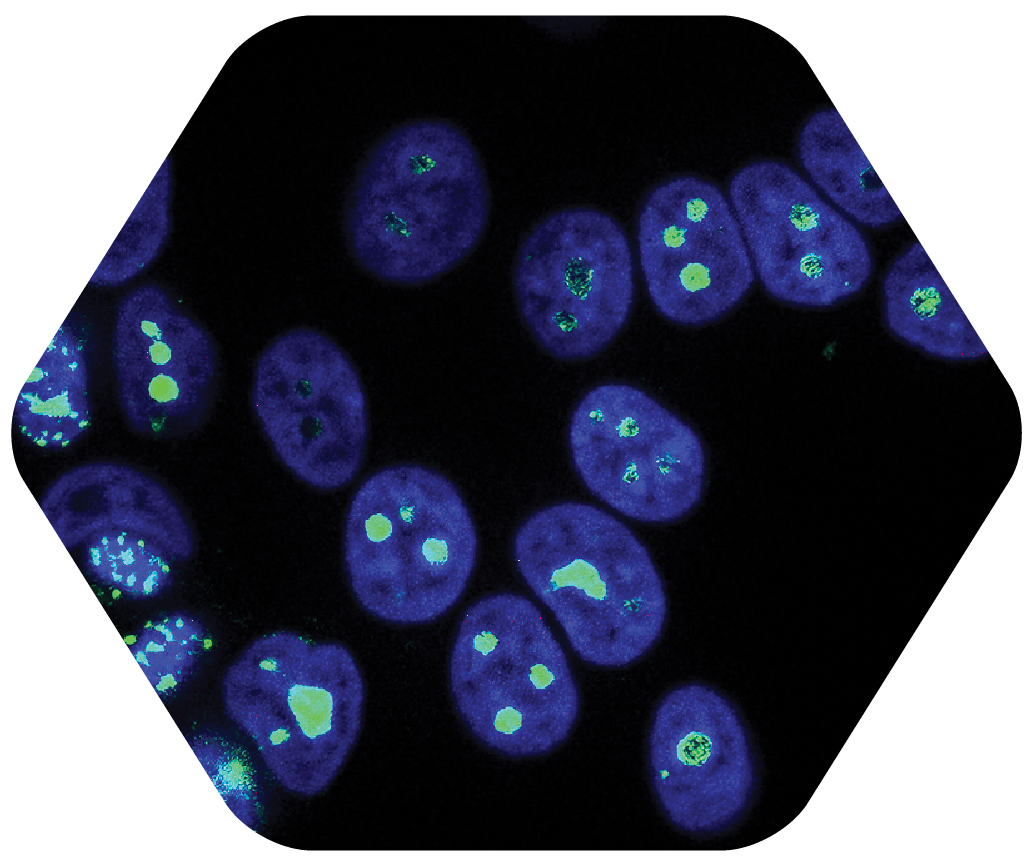
STEP 4
Step Four: Research Indicates that Microscopic Cancer Cells are Selectively Targeted with CHCP 5
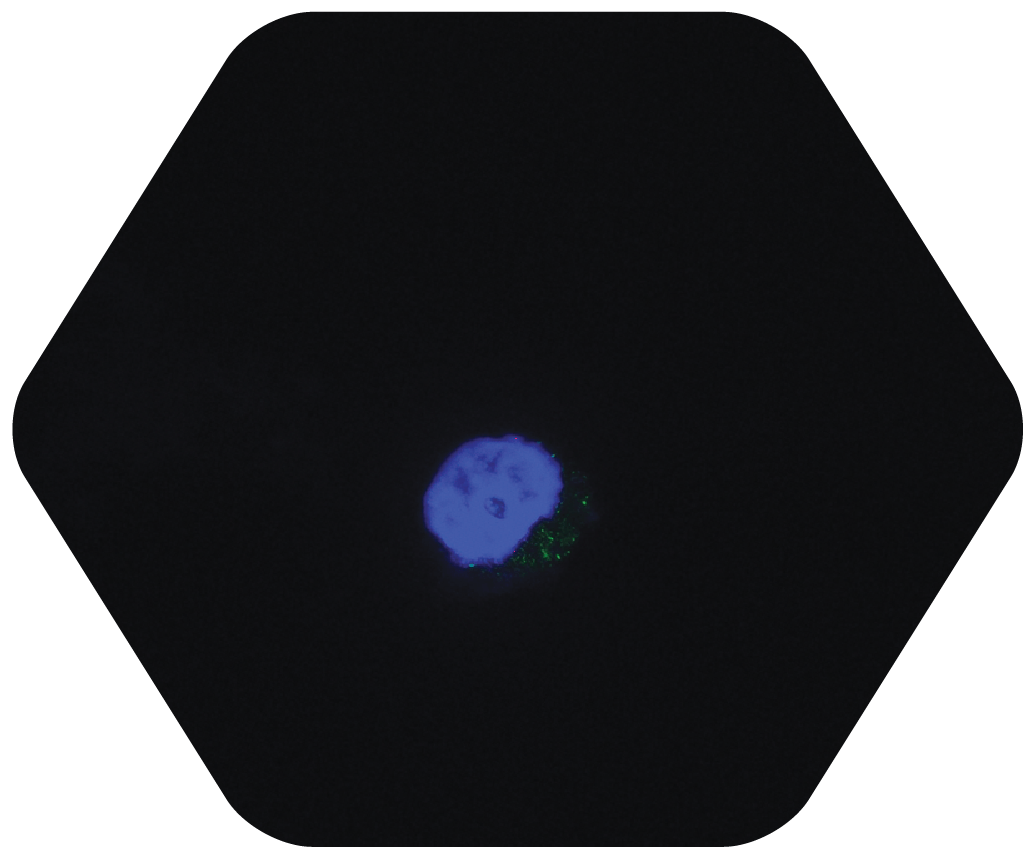
STEP 5
CHCP Interrupts the Cell Cycle of the Cancer Cell, leading to Apoptosis (cell death) 4, 5, 6, 7
4. BCL2A1 Regulates Canady Helios Cold Plasma-induced Cell Death in Triple-Negative Breast Cancer
Scientific Reports volume 12, Article number: 4038 (2022) – https://www.nature.com/articles/s41598-022-07027-4
5. Canady Helios Cold Plasma Induces Breast Cancer Cell Death by Oxidation of Histone mRNA
Int. J. Mol. Sci. 2021, 22(17), 9578; – https://doi.org/10.3390/ijms22179578
6. The synergistic effect of Canady Helios cold atmospheric plasma and a FOLFIRINOX regimen for the treatment of cholangiocarcinoma in vitro
Scientific Reports volume 11, Article number: 8967 (2021) – https://www.nature.com/articles/s41598-021-88451
7. Canady cold plasma conversion system treatment: An effective inhibitor of cell viability in breast cancer molecular subtypes
Clinical Plasma Medicine (Elsevier) Volumes 19–20, September–December 2020, 100109. – https://doi.org/10.1016/j.cpme.2020.100109
The Treatment Process

STEP 2
Tumor Removed with Canady Hybrid Plasma Scalpel

STEP 3
Tumor Margins Sprayed with Canady Helios Cold Plasma (CHCP)

STEP 5
CHCP Interrupts the Cell Cycle of the Cancer Cell, Reducing Viability, Leading to Apoptosis

STEP 1
Cancerous Tumor Located in Breast

STEP 4
Microscopic Cancer Cells are Selectively Targeted with CHCP
The Application of Canady Helios™ Cold Plasma

STEP 1
Cancerous Tumor Located in Breast

STEP 2
Tumor Removed with Canady Hybrid Plasma Scalpel

STEP 3
Tumor Margins Sprayed with Canady Helios Cold Plasma (CHCP)

STEP 4
Microscopic Cancer Cells are Selectively Targeted with CHCP

STEP 5
CHCP Interrupts the Cell Cycle of the Cancer Cell, Reducing Viability, Leading to Apoptosis
