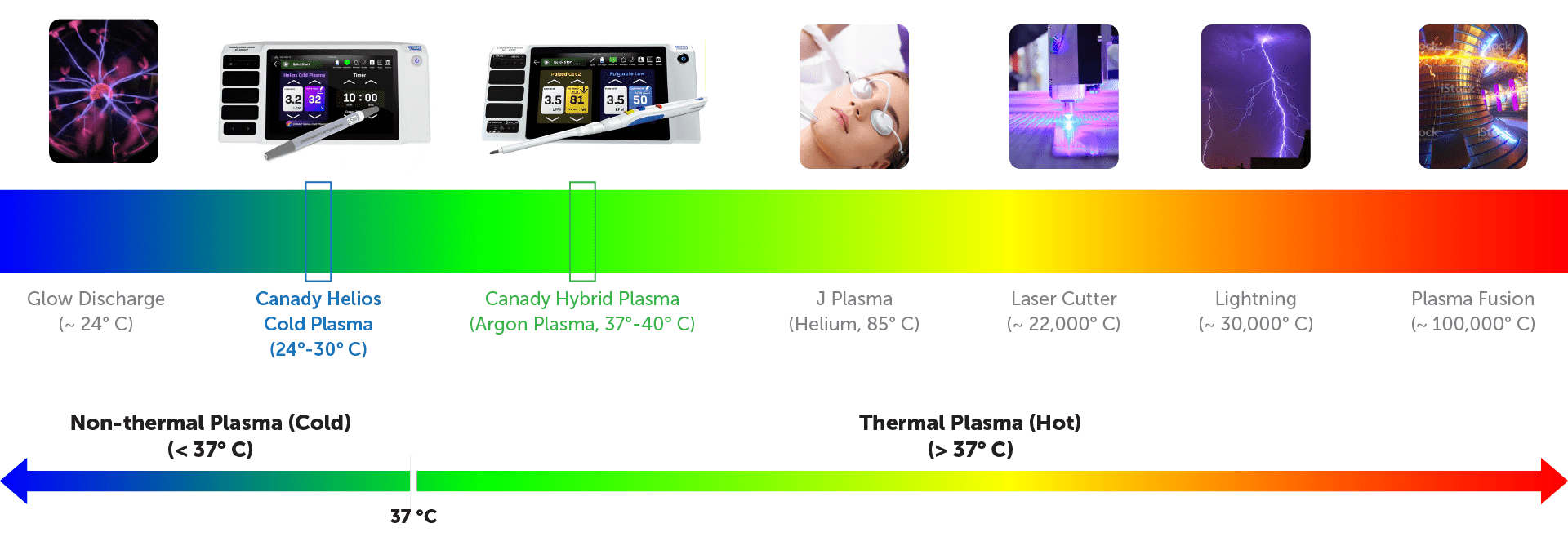
CANADY HELIOS™ COLD PLASMA

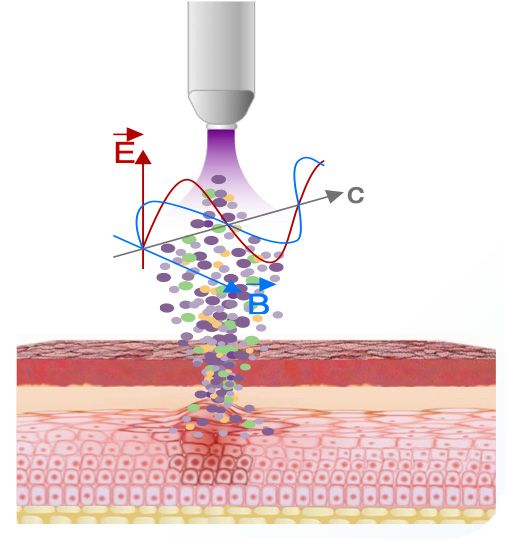
The Canady Helios Cold Plasma (CHCP) System is designed to deliver a non-thermal Cold Atmospheric Plasma (CAP) and Electromagnetic Field that targets cells, disrupts cellular homeostasis and eliminates the cell’s ability to reproduce and survive.
The CHCP System delivers a plasma beam that creates plasma species known as reactive oxygen and reactive nitrogen species (RNS and ROS) and an electromagnetic field referred to as the Plasma Treatment Electromagnetic Filed (PTEF).1
The Plasma Treatment Electromagnetic Field (PTEF)
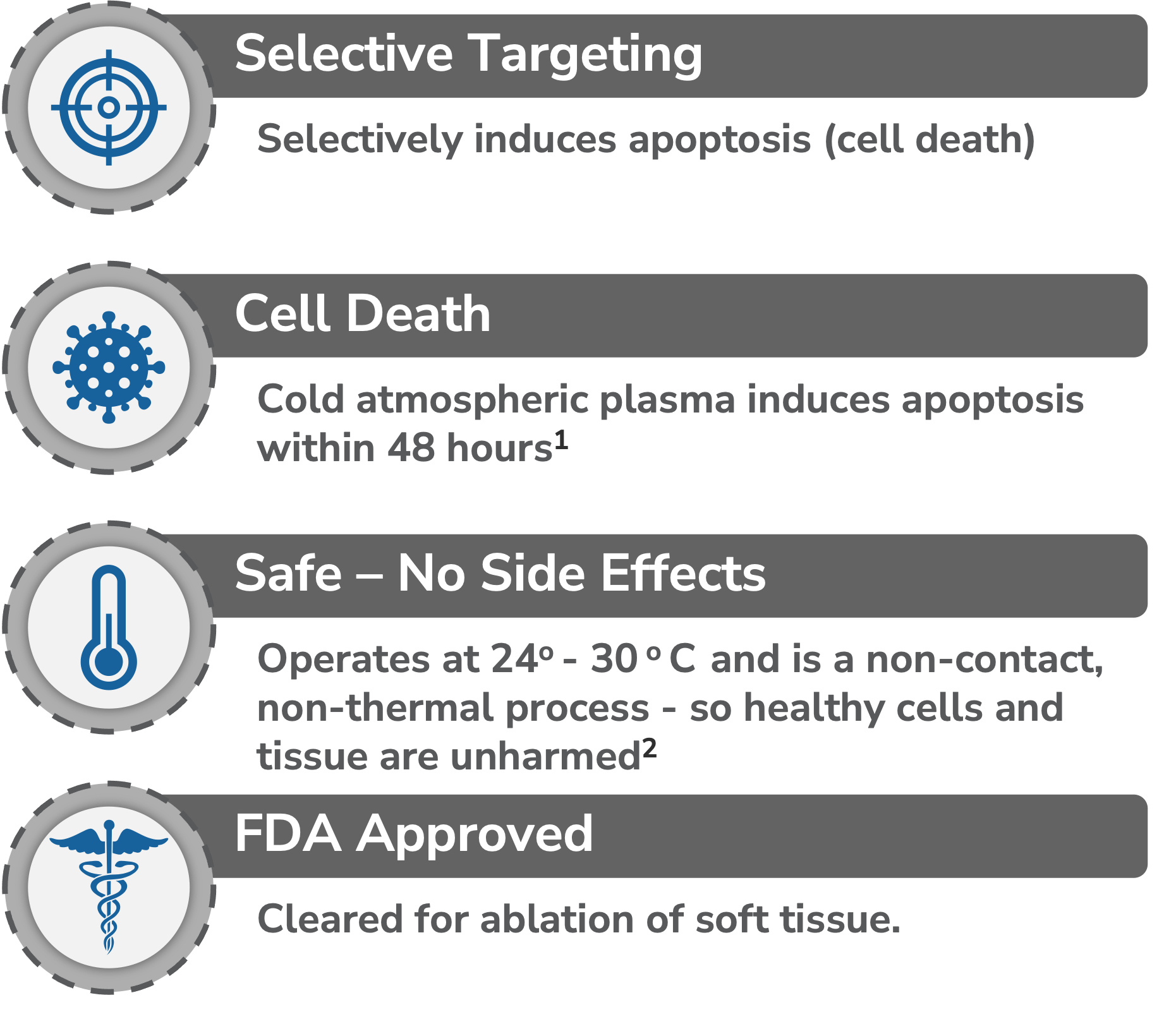
Cold Atmospheric Plasma is used to selectively target cells in the PTEF and disrupt the cell’s ability to reproduce and survive

1. Canady cold plasma conversion system treatment: An effective inhibitor of cell viability in breast cancer molecular subtypes
Clinical Plasma Medicine (Elsevier) Volumes 19–20, September–December 2020, 100109. – https://doi.org/10.1016/j.cpme.2020.100109
2. The First Cold Atmospheric Plasma Phase I Clinical Trial for the Treatment of Advanced Solid Tumors: A Novel Treatment Arm for Cancer
Cancers 2023, 15(14), 3688; – https://doi.org/10.3390/cancers15143688
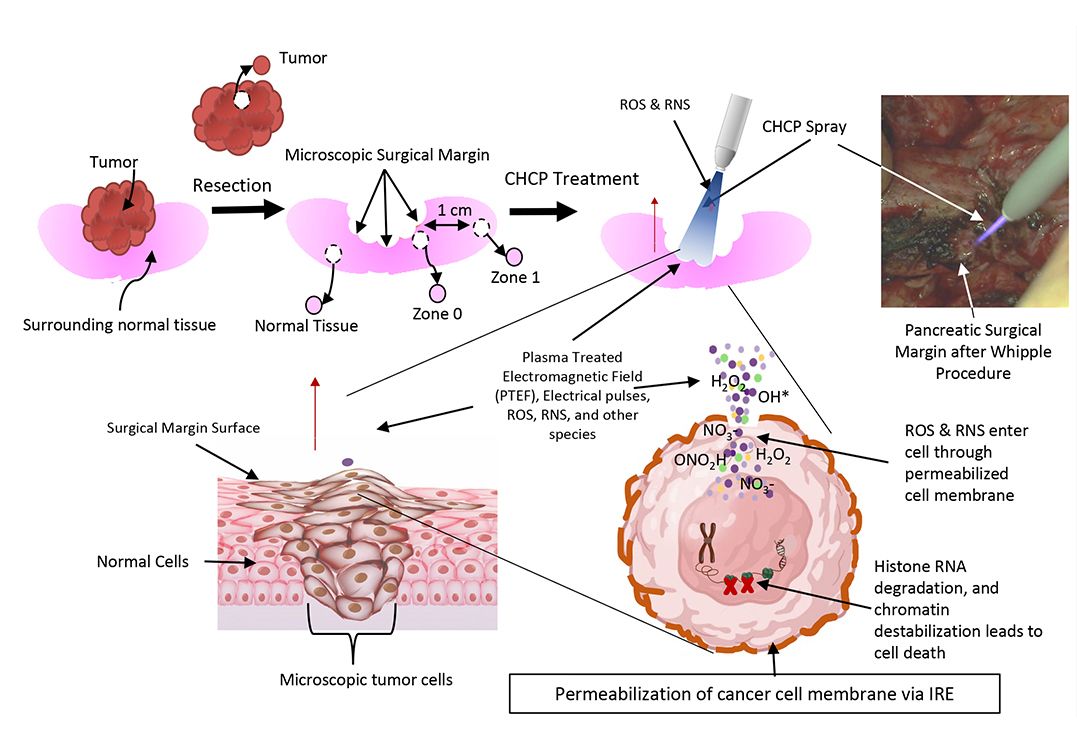
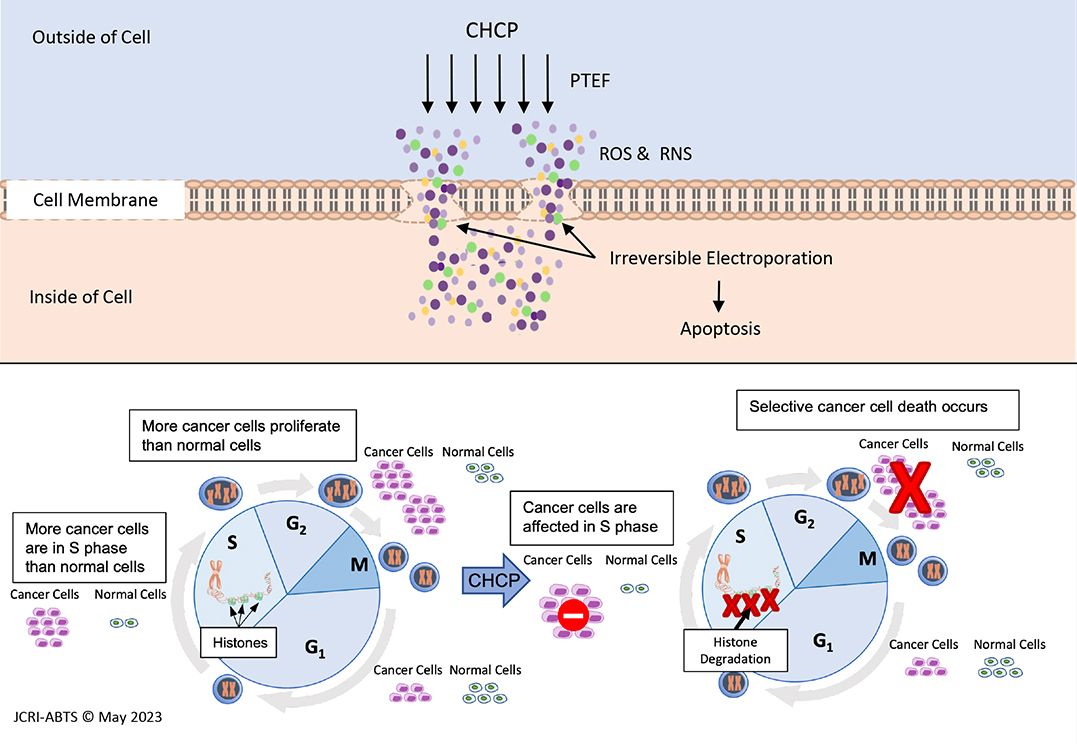
According to JCRI-ABTS Research, the PTEF created by the Plasma Spray causes Irreversible Electroporation (IRE) or openings in the cell’s membrane.
The pore formation allows the RNS and ROS to permeate the cells membrane reducing the cell’s viability leading to apoptosis (cell death).1, 2, 3, 4, 5, 7
1. The First Cold Atmospheric Plasma Phase I Clinical Trial for the Treatment of Advanced Solid Tumors: A Novel Treatment Arm for Cancer
Cancers 2023, 15(14), 3688; – https://doi.org/10.3390/cancers15143688
2. Canady Cold Helios Plasma Reduces Soft Tissue Sarcoma Viability by Inhibiting Proliferation, Disrupting Cell Cycle, and Inducing Apoptosis: A Preliminary Report
Molecules 2022, 27(13), 4168; – https://doi.org/10.3390/molecules27134168
3. The Granger Causal Effects of Canady Helios Cold Plasma on the Inhibition of Breast Cancer Cell Proliferation
Appl. Sci. 2022, 12(9), 4622; https://doi.org/10.3390/app12094622
4. BCL2A1 Regulates Canady Helios Cold Plasma-induced Cell Death in Triple-Negative Breast Cancer
Scientific Reports volume 12, Article number: 4038 (2022) – https://www.nature.com/articles/s41598-022-07027-4
5. Canady Helios Cold Plasma Induces Breast Cancer Cell Death by Oxidation of Histone mRNA
Int. J. Mol. Sci. 2021, 22(17), 9578; – https://doi.org/10.3390/ijms22179578
7. Canady cold plasma conversion system treatment: An effective inhibitor of cell viability in breast cancer molecular subtypes
Clinical Plasma Medicine (Elsevier) Volumes 19–20, September–December 2020, 100109. – https://doi.org/10.1016/j.cpme.2020.100109
The cold plasma is sprayed intraoperatively at the surgical margin after tumor removal. This process only takes 5-7 minutes.
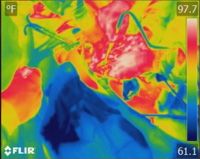
Because it’s a non-thermal process (75-86° F), normal tissue is left unharmed, so it can be used safely on organs, around ducts and vessels, and near other vital structures.
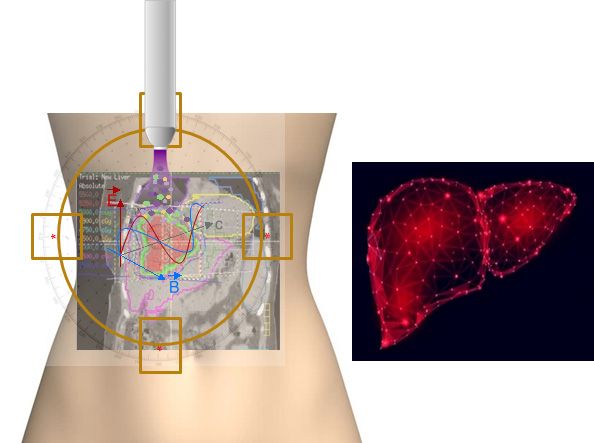
The Application of Canady Helios™ Cold Plasma
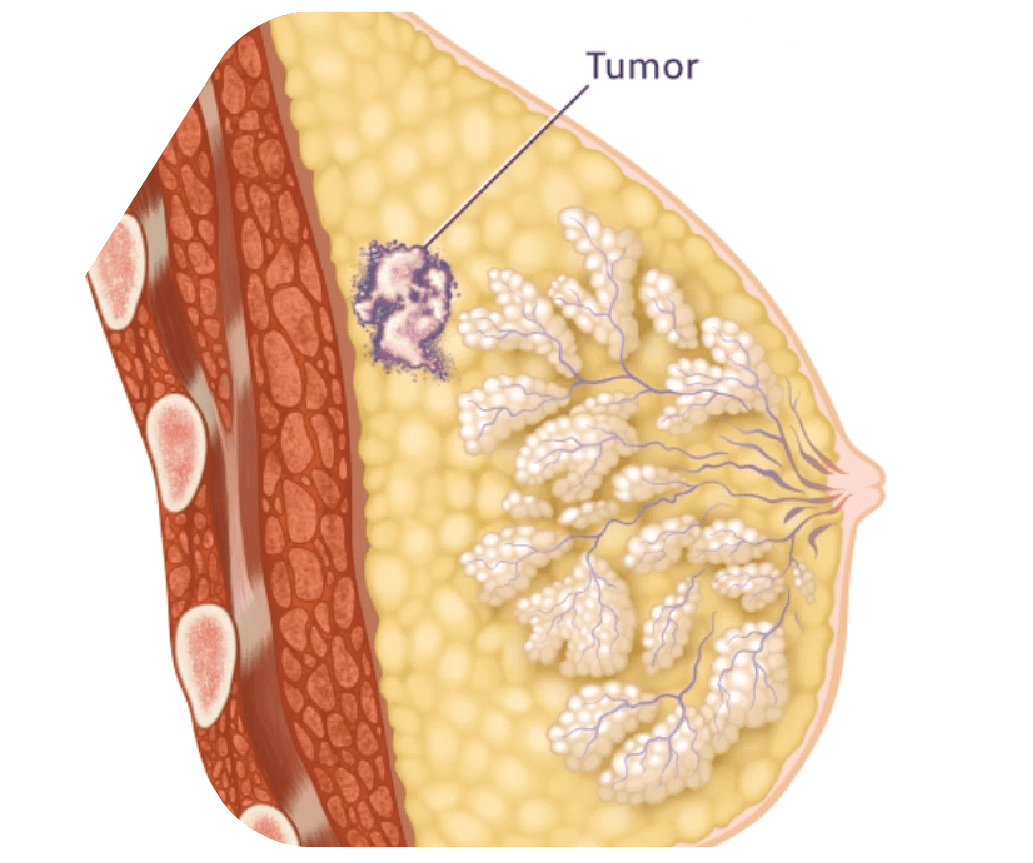
STEP 1
Cancerous Tumor Located in Breast
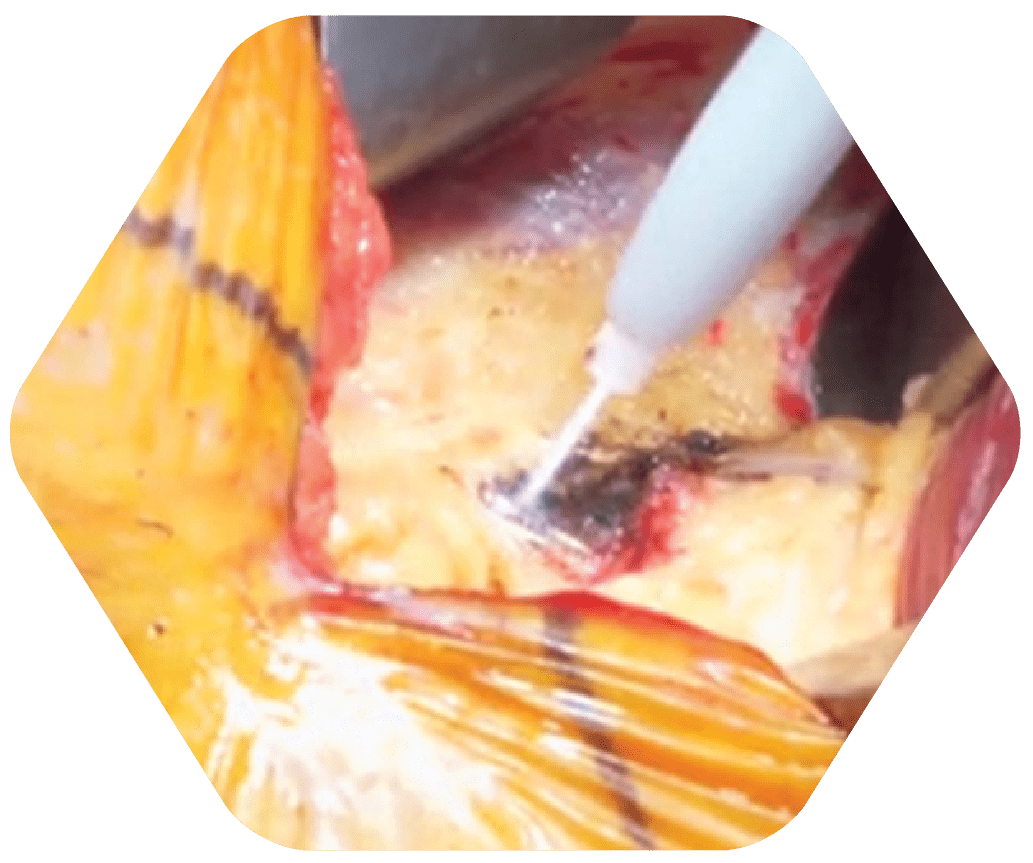
STEP 2
Tumor Removed with Canady Hybrid Plasma Scalpel
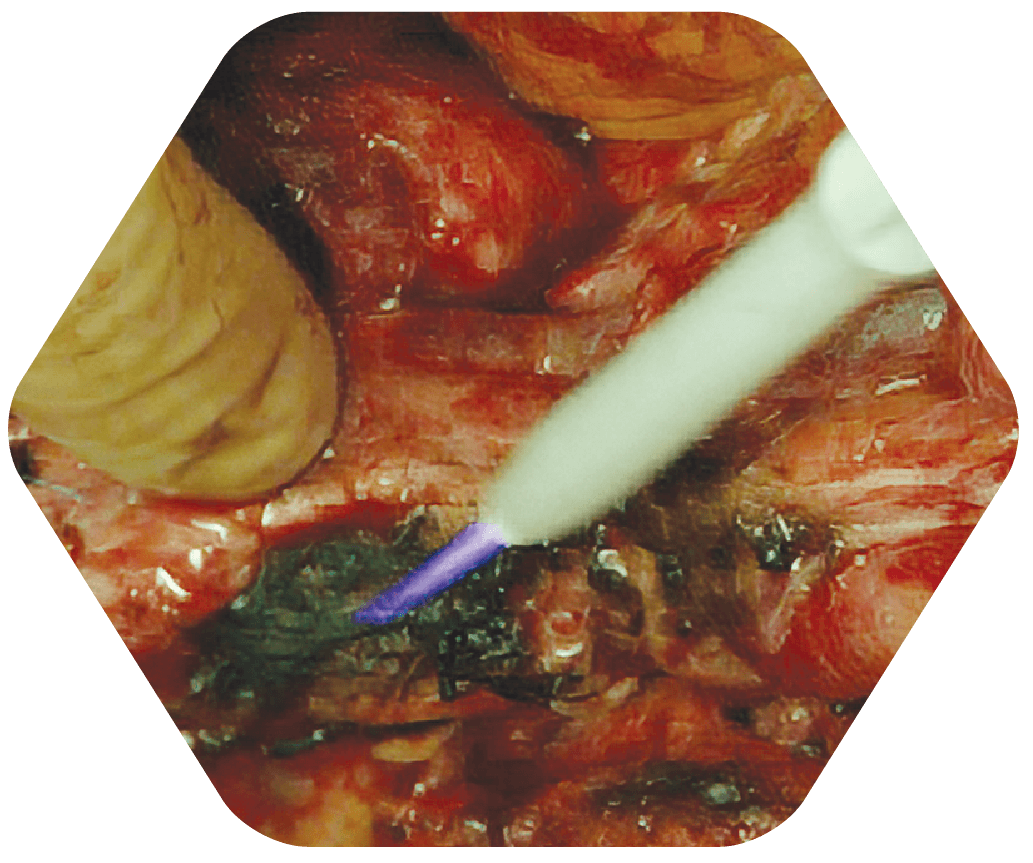
STEP 3
Tumor Margins Sprayed with Canady Helios Cold Plasma (CHCP)
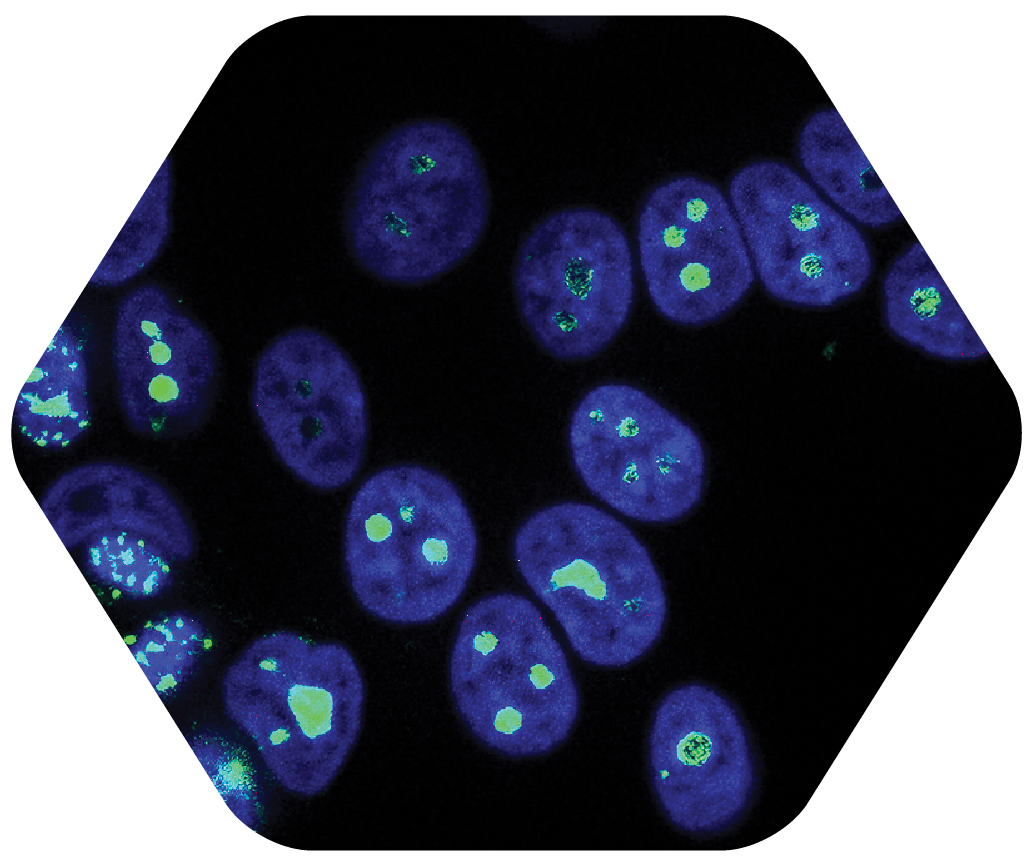
STEP 4
Step Four: Research Indicates that Microscopic Cancer Cells are Selectively Targeted with CHCP 5
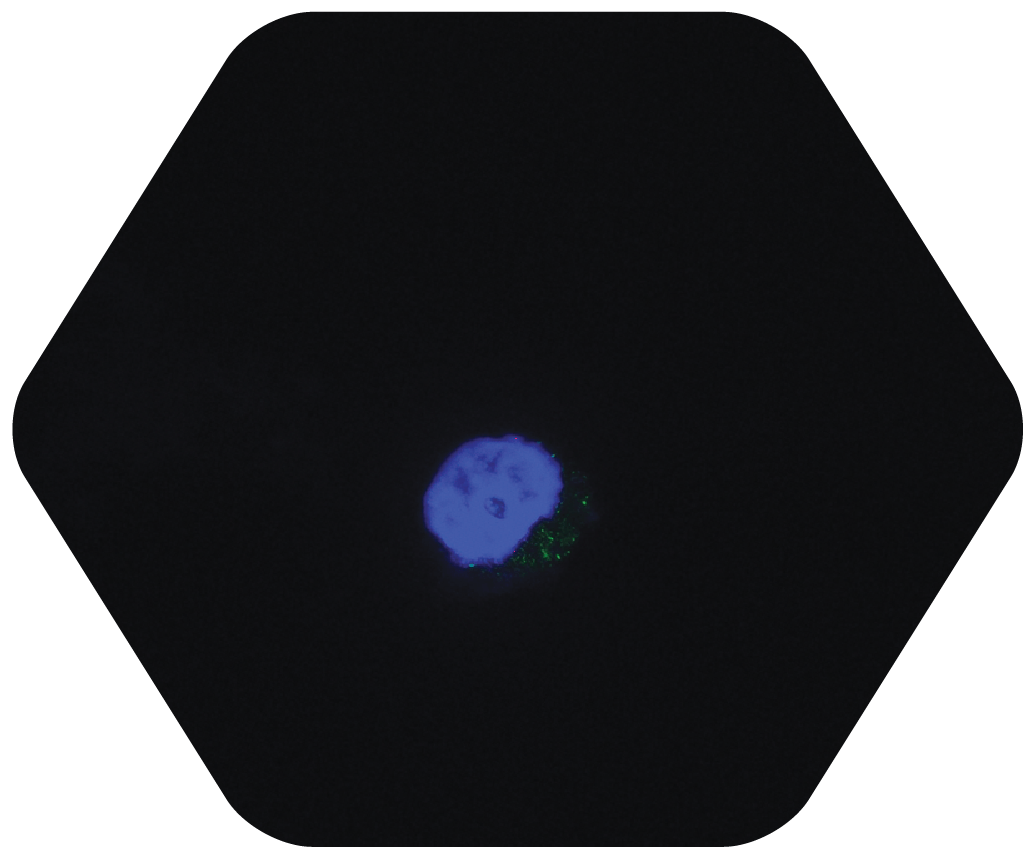
STEP 5
CHCP Interrupts the Cell Cycle of the Cancer Cell, leading to Apoptosis (cell death) 4, 5, 6, 7
4. BCL2A1 Regulates Canady Helios Cold Plasma-induced Cell Death in Triple-Negative Breast Cancer
Scientific Reports volume 12, Article number: 4038 (2022) – https://www.nature.com/articles/s41598-022-07027-4
5. Canady Helios Cold Plasma Induces Breast Cancer Cell Death by Oxidation of Histone mRNA
Int. J. Mol. Sci. 2021, 22(17), 9578; – https://doi.org/10.3390/ijms22179578
6. The synergistic effect of Canady Helios cold atmospheric plasma and a FOLFIRINOX regimen for the treatment of cholangiocarcinoma in vitro
Scientific Reports volume 11, Article number: 8967 (2021) – https://www.nature.com/articles/s41598-021-88451
7. Canady cold plasma conversion system treatment: An effective inhibitor of cell viability in breast cancer molecular subtypes
Clinical Plasma Medicine (Elsevier) Volumes 19–20, September–December 2020, 100109. – https://doi.org/10.1016/j.cpme.2020.100109
The Treatment Process

STEP 2
Tumor Removed with Canady Hybrid Plasma Scalpel

STEP 3
Tumor Margins Sprayed with Canady Helios Cold Plasma (CHCP)

STEP 5
CHCP Interrupts the Cell Cycle of the Cancer Cell, Reducing Viability, Leading to Apoptosis

STEP 1
Cancerous Tumor Located in Breast

STEP 4
Microscopic Cancer Cells are Selectively Targeted with CHCP
The Treatment Process

STEP 1
Cancerous Tumor Located in Breast

STEP 2
Tumor Removed with Canady Hybrid Plasma Scalpel

STEP 3
Tumor Margins Sprayed with Canady Helios Cold Plasma (CHCP)

STEP 4
Microscopic Cancer Cells are Selectively Targeted with CHCP

STEP 5
CHCP Interrupts the Cell Cycle of the Cancer Cell, Reducing Viability, Leading to Apoptosis
Plasma Classification