USMI Company Milestones
2024
2024

- US Medical Innovations Secures FDA Clearance for Canady Helios Cold Plasma™ Ablation System
2023
2023

- Patent Awarded for “System and Method for inducing Apoptosis in Cancer Cells”
2022
2022
- Initiated direct salesforce
- Company raised $10.2 million in a private placement
2021
2021
- Successfully completed phase 1 multi-center clinical trial using Canady Helios Cold Plasma for the treatment of cancer.
- Published successful results of Canady Helios Cold Plasma used in conjunction with FOLFIRINOX chemotherapy drug.
- Gained UL manufacturing site approval for production of XL-1000 generator.

2020
2020

- Launched Canady Plasma XL-1000 Electrosurgical Generator
- Began Canady Helios Cold Plasma Phase 1 clinical trial
- Canady Helios Cold Plasma HERO system treatment of COVID-19 collaboration with George Washington University
- Canady Helios Cold Plasma used in compassionate treatment of inoperable retroperitoneal cancer
2019
2019
- Obtained 510(k) clearance for Canady Plasma XL-1000 Electrosurgical Generator
- FDA IDE approval for Canady Helios Cold Plasma Phase 1 clinical trial in the U.S.
- Acquired Endocontrol, robotic surgical company with voice-operated robotics

2018
2018
- U.S. patents granted for Canady Helios Cold Atmospheric Plasma
2017
2017
- Canady Helios Cold Plasma and Hybrid Plasma Scalpels used in the first clinical liver tumor resection
2016
2016
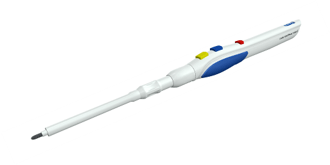
- Introduction of Paddle Blade for Canady Hybrid Plasma Scalpel
2015
2015

- Received FDA compassionate-use approval for solid tumors using Canady Helios Cold Plasma
2014
2014
- Released the 2nd generation Canady Hybrid Plasma Scalpel
- USMI designed and produced the world’s first cold plasma scalpel for cancer treatment.
2013
2013
- The world’s first Commercial Cold Plasma Generator System was developed at USMI, cold plasma research was accelerated
2012
2012
- Patent for the Method to generate Cold Plasma was granted by US Patent Office
2011
2011

- FDA approval of Canady Hybrid Plasma Scalpel, plasma generators & endoscope
- Launch of Cold Plasma Research Program
2009
2009
- USPI/USMI founded by Jerome Canady, M.D., and partners
- USPI entered into a technology transfer agreement with George Washington University (GWU) and John Hopkins University (JHU) for Cold Plasma.
